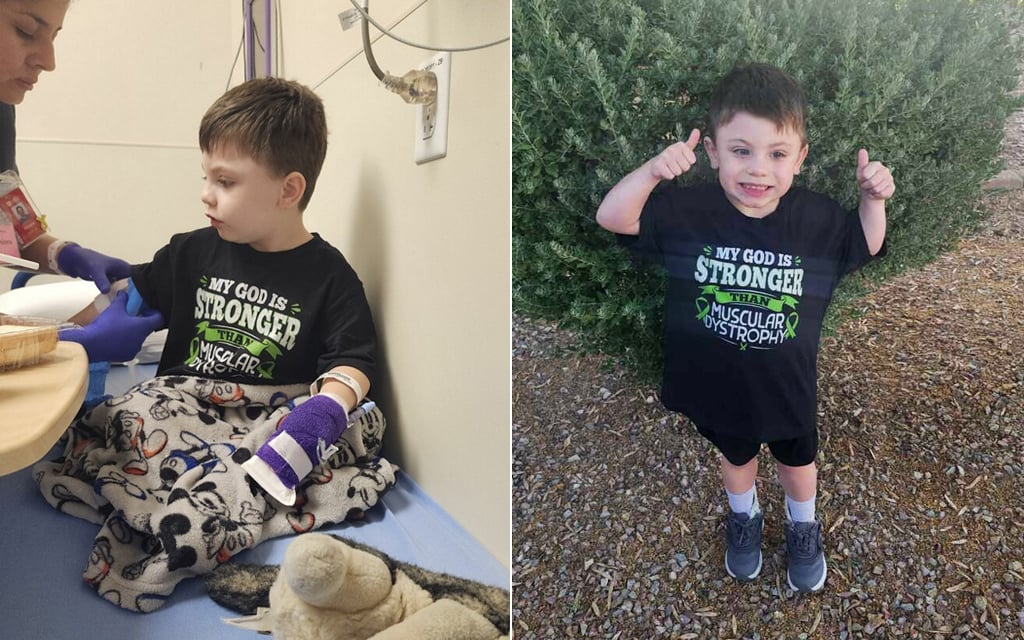
PHOENIX – Jace Taylor is a rambunctious 4-year-old boy. He runs, jumps and plays with his friends. He isn’t aware that genetically, he’s different from other children.
“I think he’s too young to comprehend what’s going on,” said Brittany Taylor, Jace’s mother. “We let him … do his little boy thing.”
Last year, Jace was diagnosed with Duchenne muscular dystrophy (DMD), a rare and fatal genetic condition that causes progressive muscle weakness and deterioration. But, in some ways, he’s lucky.
In June, the Food and Drug Administration expanded the use of Elevidys, a gene therapy, removing some of the previous restrictions to receive the treatment. Now, those ages 4 and older, who have the genetic mutation and are able to walk can receive the therapy. The FDA also conditionally approved Elevidys for those ages 4 and older who cannot walk.
The Taylors, from Buckeye, are one of five families who have had a child treated with the gene therapy at Phoenix Children’s Hospital, according to Dr. Saunder Bernes, the medical director of the hospital’s neuromuscular program.
Shortly after Jace turned 2, DMD symptoms appeared.
“Call it mother’s intuition, whatever you want to call it, but I could tell he was kind of struggling to walk,” Taylor said. “At that point, he had been walking for almost a year, and I felt like he was going backwards with walking.”

The Taylor family – from left Brittany, Jace, and Zachary – poses in Phoenix Children’s Hospital after Jace received the gene therapy. (Photo courtesy of Brittany Taylor)
The Taylors tried physical therapy, and Jace was evaluated for autism. Finally, their physical therapist recommended creatine kinase testing, which can indicate muscle disintegration.
When the test came back showing abnormal levels, they visited Bernes at Phoenix Children’s Hospital to assess whether Jace had DMD.
“At that point, I wasn’t ready, I don’t think, to know everything,” Taylor said. “It all happened so fast. … We went from, ‘Hey, Jace’s having some walking issues,’ … and it’s a lot bigger than we anticipated.”
In January 2023, Jace was officially diagnosed with DMD.
“We got the call from Dr. Bernes, his results came back positive … (and) he sent a script that day,” Taylor said. “The next day we started … steroids.”
Almost since diagnosis, the Taylors knew they would utilize the gene therapy as he was eligible under the FDA’s conditional approval.
“From day one, Dr. Bernes had mentioned to us that a) that he was pretty certain that it was going to get approved and b) that Jace would be a good candidate for it,” Taylor said. “Basically, we just had to wait for his 4th birthday.”
“We had opted to kind of wait and put all of our eggs in one basket,” she continued.
This month, Jace received the gene therapy.
What is gene therapy?
DMD results from a genetic mutation of the dystrophin gene, which holds the muscles together. When the gene is mutated, less or no dystrophin is produced. Muscles in the body, including in the heart and lungs, weaken and waste away. By their teenage years, most people affected by DMD need a wheelchair.
Disease management consists of steroids and physical therapy, along with the newly FDA-approved gene therapy. A “cocktail” approach to care is oftentimes most effective, according to Sharon Hesterlee, chief research officer of the Muscular Dystrophy Association.
Elevidys works by delivering a shortened dystrophin gene, called micro-dystrophin, into the cell, prompting the cell to produce a functional version of the dystrophin protein.
The micro-dystrophin is delivered to the cell in a noninfectious virus because of viruses’ ability to spread throughout the body.
“This is known as the one-and-done treatment … because after you give someone this treatment, it’s basically like you’ve immunized them,” Hesterlee said.
Once a person is exposed to the virus, the body creates antibodies that neutralize the virus during the next infection. In the case of Elevidys, having antibodies prevents the virus from getting into the cells and depositing the micro-dystrophin gene.
The one-time dose is administered through an IV infusion. The process can take a full day, Taylor said.
“When they told me it was a full eight-hour day with a toddler in a hospital hooked up to machines, it sounded like a nightmare,” she said. “It really wasn’t, and … you look back and think, ‘Oh my gosh, that day was kind of crazy and stressful.’”
Preparation for the therapy starts months in advance.
“We had to do the genetic testing prior to his birthday in order to submit it to insurance, just to make sure that he didn’t already have the antibodies, so we did that in March,” Taylor said. “On his birthday (in) April … they submitted to insurance, and then we got the approval in a month.”
The Taylors are grateful for the treatment.
“In perspective, I would much rather have him do a one-time treatment that has so much positive for it than have to go every three months like some of the other conditions,” Taylor said.
The disease affects one in 3,500 to 5,000 male newborns worldwide. Because the mutation lies on the X chromosome, more boys are affected than girls. Those with DMD often die by their mid-20s.
Hope of a longer lifespan shadowed by fears

Phoenix Children’s Hospital houses a neuromuscular program that includes care for those with Duchenne muscular dystrophy. Dr. Saunder Bernes heads the unit. (Photo courtesy of Phoenix Children’s Hospital)
Though Elevidys is not a cure, it is a step forward in the treatment of a fatal condition that currently has few options.
“The hope is that we will change the trajectory of this disorder,” said Bernes of Phoenix Children’s Hospital.
When the Taylors were going through the diagnosis, the gene therapy was the light at the end of the tunnel “going into something that was super serious and scary,” Taylor said.
“From day one, his doctor was ready to get him on the gene therapy,” she said. “It was good timing, I guess, for us to get the diagnosis because they had all of this already in the works.”
The focus of DMD therapies is on stabilizing or slowing progression, allowing for patients to live a longer and more comfortable life.
“Normally, we would see significant deterioration over a rapid period of time between about 7 ½ to about 10 or 11,” Bernes said. “If we can prolong that and put it into the teenage years, we’ll see boys who survive now into late adulthood.”
But, the longevity of the treatment is a concern for providers and patients.
“We don’t know as much about what the risks are or the benefits because as you get older with this disease, you lose more and more of your muscle,” Hesterlee said. “You can’t put new genes in muscle that doesn’t exist.”
Jill Castle is the director of education and patient services for the Little Hercules Foundation, a nonprofit advocacy group focused on rare diseases.
“They’ve only been in trial for a handful of years, so we don’t know what this is going to look like on a boy in 10 years,” she said. “It’s slowing the progression … and we don’t have a treatment yet to give after it wears off.”
Disease management options for children who make it into adulthood decrease significantly, highlighting a gap in research. Castle, whose 24-year-old son Anthony has DMD, is traversing uncharted territory as her son continues to age.

Jill Castle committed herself to helping other parents whose children have Duchenne muscular dystrophy after her son, Anthony, received his diagnosis of DMD about 20 years ago. (Photo courtesy of Jill Castle)
“Anthony’s had interventions that nobody’s ever had before,” Castle said, noting after years of treatments, her son’s quality of life has severely diminished.
“If I, at this point, in trying to extend his life 10 more years, is it for me or is it for him? What is that 10 years going to look like?” she said. “My son’s in a wheelchair, has braces, has contractures, has pain. He’s on a breathing machine during the day that he sips so he can talk and have energy, he’s on a breathing machine at night. … I have to intervene on bowel stuff every few days because he can’t go on his own anymore. That is not something I’m going to try to keep going for 20 years just to say he’s here.”
Castle and her family decided that her son would not receive Elevidys because he is at the end stages of the disease. Though he is eligible under the expanded age range, those under 4 are not.
An issue treating infants and toddlers with gene therapy is that symptoms typically arise between the ages of 3 and 6. But, Castle argued that newborn screening for DMD would allow parents to know early on.
“With the benefit of newborn screening, the next generation of families will have even more time to evaluate, pursue and access FDA-approved Duchenne therapies while avoiding extensive surgeries, limitations and suffering,” she wrote in an op-ed for the Arizona Republic.
Bernes agreed that newborn screening increases the “possibility of treating patients earlier.”
Though there is no requirement for DMD newborn screening in Arizona, Castle has been active in lobbying for DMD to be added to the list and advocated for SB 1020 this year, which did not advance in the Arizona Legislature.
In 2023, Ohio became the first state to mandate DMD be included in newborn screenings with New York following. In January, Minnesota also added DMD to screening requirements.
Concerns surround lack of data, cost
An Elevidys treatment, the only gene therapy available for DMD, is estimated to cost $3.2 million, but the majority of the burden falls on the insurance provider.
“I assumed it was going to be a nightmare, but they didn’t give any problems, and we had no out-of-pocket costs,” said Taylor, who was able to have the treatment covered by insurance. “We were really fortunate for that.”
Castle helps those with DMD navigate the complexities of insurance coverage, including through Medicaid.
“If you did a disease-cost analysis … over their lifetime, it’s considerably more than $3.5 million,” Castle said. “In the long run, it is going to save money.”
For current treatment and management, the costs quickly rack up.
“My son has a wheelchair that’s $70 grand, and he needs one every five years, and he needs breathing machines and standing equipment and he’s had back surgery,” Castle said. “When you look at all those costs, you can see that gene therapy then turns out to make more sense.”
The process can also be a drain on hospital resources, according to Bernes. Phoenix Children’s Hospital even had an employee to distract Jace with an iPad while receiving the therapy.
“It takes a huge village to treat one patient efficiently and safely,” he said. “There’s somebody who needs to be able to coordinate all of this, and then we need to be able to follow these patients.”
Concerns also arise around a lack of data.
For children ages 4 to 5 who can walk and have a proven DMD mutation, Elevidys was conditionally approved in 2023 for serious or life-threatening conditions until more data was gathered. After a year of conditional approval, the FDA approved Elevidys for those 4 and older who can walk.
Elevidys is only conditionally approved for those 4 and older who cannot walk, highlighting the lack of data from clinical trials regarding nonambulatory children.
“It was kind of a big deal that they expanded it to nonambulatory boys because we don’t have as much data there,” said Hesterlee of the Muscular Dystrophy Association. “It’s hard to do a risk-benefit analysis because we really don’t know what the benefit will be or the risk.”
Because so few patients have received the therapy in general, long-term side effects have yet to be discovered.

Jace Taylor, 4, proudly throws up a thumbs-up after receiving gene therapy treatment in Phoenix Children’s Hospital. (Photo courtesy of Brittany Taylor)
“There haven’t been thousands of boys treated with this – we’re talking about the hundreds,” Bernes said. “Any type of side effect, it may take thousands of boys to be treated before we see adverse effects.”
But, the potential benefits greatly outweigh the potential risks, Hesterlee said.
“I think the FDA came down on the side of saying, we need to give people a choice. We need to give them access. This is a fatal disease,” she said.
“When you’re looking at a disease like Duchenne, the boys are going to pass away anyway,” Castle added. “We’re still going to lose our boys if we don’t take the risks.”
Taylor’s concerns center around next steps.
“It’s not a cure, it’s a treatment,” she said. “At the point where he may potentially start going downhill, is there going to be an option for another dose?”
Despite a lack of data, the Taylors went ahead with the treatment.
“My goal, ultimately, is making sure in the time that Jace is here on Earth that he’s able to do as much as he can, as a typical boy, young man,” Taylor said, “being able to live life while he can.”
Jace has always been an active boy, but since the gene therapy, he’s a ball of energy.
“His neurologist … she was really amazed by what Jace can do, and that is confirmation to us that we did the right thing,” Taylor said. “For somebody who knows what they’re looking for and for them to say, ‘If I were to see him on the street, I would never imagine that he has Duchenne,’ is a big deal for me. I’ve got confirmation that we’re doing everything that we possibly can for Jace.”